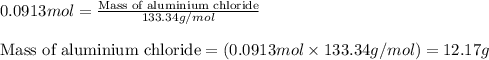Chemistry, 20.03.2020 00:04 janessa0804
Find the mass of AlCl3 that is produced when 10.0 grams of Al2O3 react with 10.0 g of HCl according to the following equation. Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Find the mass of AlCl3 that is produced when 10.0 grams of Al2O3 react with 10.0 g of HCl according...
Questions



English, 11.12.2020 08:40

Geography, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

Health, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40


History, 11.12.2020 08:40

Biology, 11.12.2020 08:40



Chemistry, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

English, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

Mathematics, 11.12.2020 08:40

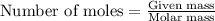 .....(1)
.....(1)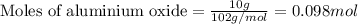
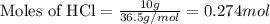
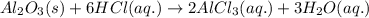
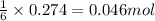 of aluminium oxide
of aluminium oxide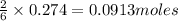 of aluminium chloride
of aluminium chloride