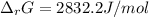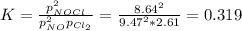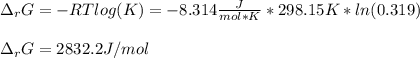Chemistry, 20.03.2020 00:06 nguyenhoangthienkim0
A chemist fills a reaction vessel with 9.47 atm nitrogen monoxide (NO) gas, 2.61 atm chlorine (C12) gas, and 8.64 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction:
2NO(g) + Cl2(g) <=> 2NOCI (g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
A chemist fills a reaction vessel with 9.47 atm nitrogen monoxide (NO) gas, 2.61 atm chlorine (C12)...
Questions



Mathematics, 02.12.2020 01:30


Mathematics, 02.12.2020 01:30

History, 02.12.2020 01:30

Mathematics, 02.12.2020 01:30

Mathematics, 02.12.2020 01:30



Mathematics, 02.12.2020 01:30



Mathematics, 02.12.2020 01:30


Mathematics, 02.12.2020 01:30

SAT, 02.12.2020 01:30

Mathematics, 02.12.2020 01:30

Mathematics, 02.12.2020 01:30