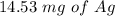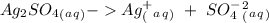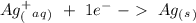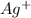Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Suppose a current of 250.mA is passed through an electroplating cell with an aqueous solution of Ag2...
Questions



Advanced Placement (AP), 03.08.2020 14:01

Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01






Mathematics, 03.08.2020 14:01

Computers and Technology, 03.08.2020 14:01

Business, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01


Chemistry, 03.08.2020 14:01

Biology, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01