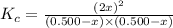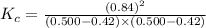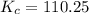Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1


Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction e...
Questions







Mathematics, 09.09.2020 09:01


Chemistry, 09.09.2020 09:01


Mathematics, 09.09.2020 09:01

Mathematics, 09.09.2020 09:01

Mathematics, 09.09.2020 09:01

English, 09.09.2020 09:01

Mathematics, 09.09.2020 09:01

Mathematics, 09.09.2020 09:01

Mathematics, 09.09.2020 09:01

Biology, 09.09.2020 09:01

History, 09.09.2020 09:01

Chemistry, 09.09.2020 09:01

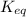 is 110.25
is 110.25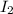 = 0.500 mole
= 0.500 mole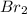 = 0.500 mole
= 0.500 mole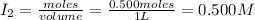
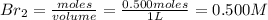
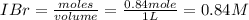 [/tex]
[/tex]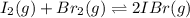
![K_c=\frac{[IBr]^2}{[Br_2]\times [I_2]}](/tpl/images/0554/7930/da4ed.png)