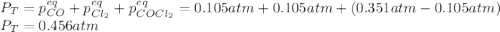CO(g) + Cl2 (g) ⇌ COCl2 (g) Kp = 22.5 at 395 °C . A sample of COCl2 (g) is introduced into a constantvolume vessel at 395 °C and observed to exert an initial pressure of 0.351 atm. When equilibrium is established, what will be the total pressure within the vessel?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
CO(g) + Cl2 (g) ⇌ COCl2 (g) Kp = 22.5 at 395 °C . A sample of COCl2 (g) is introduced into a constan...
Questions

Chemistry, 02.12.2020 01:00




English, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00

World Languages, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00

Computers and Technology, 02.12.2020 01:00

Social Studies, 02.12.2020 01:00

English, 02.12.2020 01:00






Chemistry, 02.12.2020 01:00

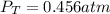
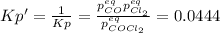
 due to reaction's progress, one obtains:
due to reaction's progress, one obtains: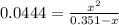
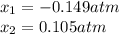
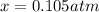 for which the total pressure at equilibrium is:
for which the total pressure at equilibrium is: