
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1


Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts...
Questions


Mathematics, 27.12.2019 08:31

Mathematics, 27.12.2019 08:31

Mathematics, 27.12.2019 08:31

Mathematics, 27.12.2019 08:31


Computers and Technology, 27.12.2019 08:31



Health, 27.12.2019 08:31




English, 27.12.2019 09:31






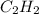 gas is often used in welding torches because of the very high heat produced when it reacts with oxygen
gas is often used in welding torches because of the very high heat produced when it reacts with oxygen 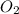 gas, producing carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 1.5 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
gas, producing carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 1.5 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.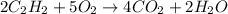
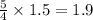 moles of oxygen
moles of oxygen

