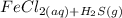Chemistry, 19.03.2020 21:27 Hamadsaqer9
Solid iron(II) sulfide reacts with aqueous hydrochloric acid HCl to produce hydrogen sulfide gas H2S and aqueous iron(II) chloride . Write a balanced chemical equation for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2


Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Solid iron(II) sulfide reacts with aqueous hydrochloric acid HCl to produce hydrogen sulfide gas H2S...
Questions

Chemistry, 28.01.2020 16:42

Physics, 28.01.2020 16:42


Computers and Technology, 28.01.2020 16:42



Geography, 28.01.2020 16:42



Mathematics, 28.01.2020 16:42

Biology, 28.01.2020 16:42

Social Studies, 28.01.2020 16:43




Biology, 28.01.2020 16:43


History, 28.01.2020 16:43

Mathematics, 28.01.2020 16:43

History, 28.01.2020 16:43