Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Consider the reaction for the combustion of benzene: 2 C6H6(g) 15 O2(g) ---> 12 CO2(g) 6 H2O(l) W...
Questions

Mathematics, 09.11.2021 21:10





Mathematics, 09.11.2021 21:10

Mathematics, 09.11.2021 21:10



Biology, 09.11.2021 21:10

History, 09.11.2021 21:10


Biology, 09.11.2021 21:10



Chemistry, 09.11.2021 21:10

English, 09.11.2021 21:10

Mathematics, 09.11.2021 21:10

English, 09.11.2021 21:10

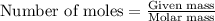 .....(1)
.....(1)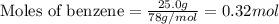
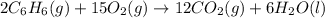
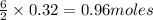 of water
of water