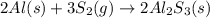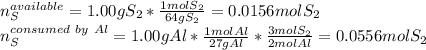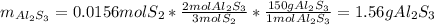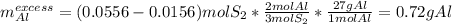Chemistry, 19.03.2020 21:45 2023jpeterson
Aluminum reacts with sulfur gas to produce aluminum sulfide. a) What is the limiting reactant? What is the excess reagent? b) How many grams of Aluminum Sulfide will be produced? c) How many grams of the excess reactant will be left over in the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1
You know the right answer?
Aluminum reacts with sulfur gas to produce aluminum sulfide. a) What is the limiting reactant? What...
Questions


Biology, 29.09.2021 09:30

English, 29.09.2021 09:30



Mathematics, 29.09.2021 09:40



Mathematics, 29.09.2021 09:40

Mathematics, 29.09.2021 09:40


Mathematics, 29.09.2021 09:40

Mathematics, 29.09.2021 09:40

World Languages, 29.09.2021 09:40

Mathematics, 29.09.2021 09:50

Mathematics, 29.09.2021 09:50


Mathematics, 29.09.2021 09:50


Mathematics, 29.09.2021 14:00