
Chemistry, 19.03.2020 20:33 tfaulk2884
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) In an experiment, 201 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 732torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is 26.74 torr.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1


Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) I...
Questions


History, 13.11.2020 08:10

Mathematics, 13.11.2020 08:10


Mathematics, 13.11.2020 08:10


English, 13.11.2020 08:10


History, 13.11.2020 08:10

Geography, 13.11.2020 08:10

Biology, 13.11.2020 08:10

Mathematics, 13.11.2020 08:10

Mathematics, 13.11.2020 08:10



Mathematics, 13.11.2020 08:10


Biology, 13.11.2020 08:10


History, 13.11.2020 08:10

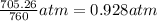
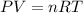
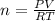
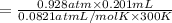
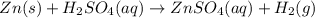
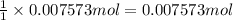 of zinc
of zinc

