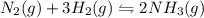Chemistry, 19.03.2020 20:43 lovelylife7553
The reaction for the synthesis of ammonia N2(g) + 3 H2(g) → 2 NH3(g) is exothermic. Increasing the temperature applied to the system I) increases the amount of NH3. II) decreases the amount of NH3. III) changes the value of Keq. IV) does not change the value of Keq.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
The reaction for the synthesis of ammonia N2(g) + 3 H2(g) → 2 NH3(g) is exothermic. Increasing the t...
Questions






Mathematics, 07.12.2020 21:00



Health, 07.12.2020 21:00



Biology, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00



Mathematics, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00

Geography, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00