
You're provided with a bottle labled [CoCl2.6H2O] = 0.056 M in 4.60 M HCl. You heat a small volume of the solution in a hot water bath to 50 ∘C. If you determine that [CoCl42-] = 0.031 M at 50 ∘C at equilibrium, what must be the equilibrium concentration of [Cl-] at 50 ∘C?

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2

You know the right answer?
You're provided with a bottle labled [CoCl2.6H2O] = 0.056 M in 4.60 M HCl. You heat a small volume o...
Questions

English, 20.10.2020 04:01


English, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01










Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01




![[CoCl4^{-2}] = x = 0.031 M](/tpl/images/0554/3885/8cf6f.png)
![[Cl^{-}] = (4.60 - 2*0.031) M = 4.54 M](/tpl/images/0554/3885/d2bfa.png)
![[CoCl_{2}\cdot 6H_{2}O] = (0.056 - 0.031) M = 0.025 M](/tpl/images/0554/3885/f4e13.png)
![[Cl^-]](/tpl/images/0554/3885/0726e.png) at
at 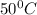 is 4.538 M
is 4.538 M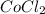 = 0.056 M
= 0.056 M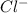 = 4.60 M
= 4.60 M![COCl_2+2Cl^-\rightleftharpoons [CoCl_4]^{2-}+6H_2O](/tpl/images/0554/3885/5e05e.png)
![K_c=\frac{[CoCl_4]^{2-}\times [H_2O]^6}{[CoCl_2]^2\times [Cl^-]^2}](/tpl/images/0554/3885/4ddc1.png)
![[CoCl_4]^{2-}](/tpl/images/0554/3885/84955.png) =x = 0.031 M
=x = 0.031 M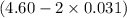 =4.538 M
=4.538 M ![You're provided with a bottle labled [CoCl2.6H2O] = 0.056 M in 4.60 M HCl. You heat a small volume o](/tpl/images/0554/3885/222c5.jpg)


