
The decomposition of ozone in the upper atmosphere to dioxygen occurs by a two-step mechanism. The first step is a fast reversible step and the second is a slow reaction between an oxygen atom and an ozone molecule:Step 1: O3(g) O2(g) + O(g) Fast, reversible, reactionStep 2: O3(g) + O(g) → 2O2(g) SlowWhich is the rate determining step?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
The decomposition of ozone in the upper atmosphere to dioxygen occurs by a two-step mechanism. The f...
Questions

History, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00

Computers and Technology, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00


Biology, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00






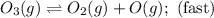
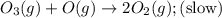
![\text{Rate}=k[O_3][O]](/tpl/images/0554/3771/ba34f.png)



