Chemistry, 19.03.2020 17:08 Levantine3667
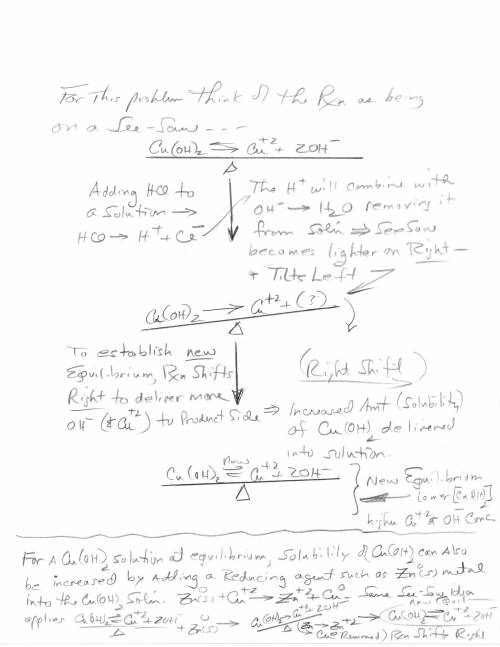
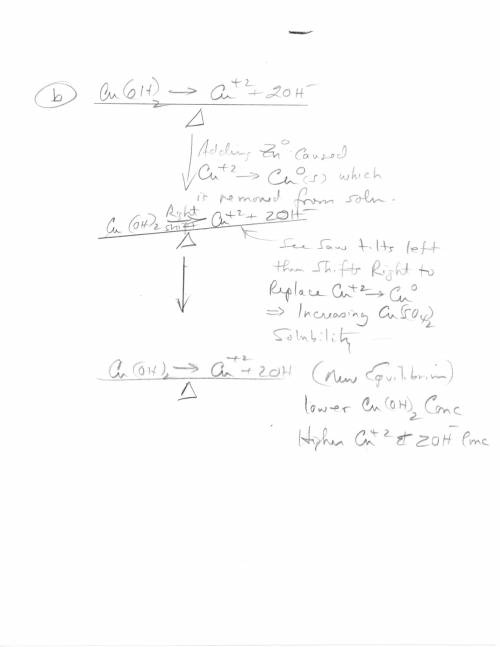
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly soluble. Cu(OH)2(s) Cu2 (aq) 2OH- (aq) a. Explain how the solubility can be increased by adding HCl to the solution. b. Explain how the concentration of copper (II) ion or of hydroxide ion can be reduced in the solution so that more of the solid copper hydroxide can be dissolved.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

You know the right answer?
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly...
Questions




Mathematics, 10.02.2021 19:20

Chemistry, 10.02.2021 19:20


Mathematics, 10.02.2021 19:20

History, 10.02.2021 19:20


Mathematics, 10.02.2021 19:20


English, 10.02.2021 19:20


Mathematics, 10.02.2021 19:20

History, 10.02.2021 19:20


Social Studies, 10.02.2021 19:20

Advanced Placement (AP), 10.02.2021 19:20

Chemistry, 10.02.2021 19:20