Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

You know the right answer?
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0144 0.0144 M solution. The pH...
Questions

Biology, 07.11.2020 01:00


Mathematics, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

History, 07.11.2020 01:00

Biology, 07.11.2020 01:00

English, 07.11.2020 01:00


Mathematics, 07.11.2020 01:00

English, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

Social Studies, 07.11.2020 01:00



Mathematics, 07.11.2020 01:00





 .
.![pH=-\log[H^+]](/tpl/images/0554/0412/cf945.png)
![2.46=-\log[H^+]](/tpl/images/0554/0412/98673.png)
![[H^+]=0.003467 M](/tpl/images/0554/0412/5a2c6.png)
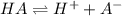
![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0554/0412/a5cb9.png)
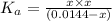
![x=[H^+]=0.003467 M](/tpl/images/0554/0412/5abc8.png)