Chemistry, 19.03.2020 09:31 priscillarios30
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g) Calculate the equilibrium concentrations of reactant and products when 0.372 moles of HI are introduced into a 1.00 L vessel at 698 K. [HI] = M [H2] = M [I2] = M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g)...
Questions

Mathematics, 06.03.2020 22:16

Physics, 06.03.2020 22:16


Biology, 06.03.2020 22:16


English, 06.03.2020 22:16

Social Studies, 06.03.2020 22:16




Mathematics, 06.03.2020 22:17




Geography, 06.03.2020 22:17





![[HI]=(0.372-2x) M =(0.372-2\times 0.03935)M =0.2933 M](/tpl/images/0553/9717/216fb.png)
![[H_2]=x = 0.03935 M](/tpl/images/0553/9717/0d5d5.png)
![[I_2]=x = 0.03935 M](/tpl/images/0553/9717/d2ad6.png)
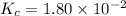
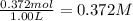
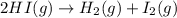
![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0553/9717/ef85e.png)