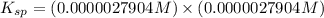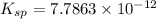Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
. Calculate Ksp for copper oxalate (CuC2O4). Use your calculated copper ion concentration (0.0000027...
Questions



Mathematics, 14.05.2021 20:50

English, 14.05.2021 20:50


Mathematics, 14.05.2021 20:50


English, 14.05.2021 20:50

Mathematics, 14.05.2021 20:50


Mathematics, 14.05.2021 20:50

Mathematics, 14.05.2021 20:50

Social Studies, 14.05.2021 20:50


English, 14.05.2021 20:50


Mathematics, 14.05.2021 20:50

Mathematics, 14.05.2021 20:50

English, 14.05.2021 20:50

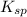 is
is 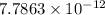
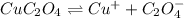
![K_{sp}=[Cu^{+}][C_2O_4^{-}]](/tpl/images/0553/9563/c6650.png)