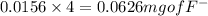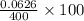A 0.400-g sample of toothpaste was boiled with a 50-mL solution containing a citrate buffer and NaCl to extract a fluoride ion. After cooling, the solution was diluted to exactly 100 mL. The potential of ISE with Ag-AgCl (sat) reference electrode in a 25.0-mL aliquote of the sample was found to be -0.1823 V. Addition of 5.0 mL of a solution containing 0.00107 mg F-/mL caused the potential to change to -0.2446 V. Calculate the percentage of F- in the sample.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
A 0.400-g sample of toothpaste was boiled with a 50-mL solution containing a citrate buffer and NaCl...
Questions

Mathematics, 11.07.2019 09:30

Mathematics, 11.07.2019 09:30

Social Studies, 11.07.2019 09:30


Biology, 11.07.2019 09:30



Mathematics, 11.07.2019 09:30

Mathematics, 11.07.2019 09:30



Mathematics, 11.07.2019 09:30



Advanced Placement (AP), 11.07.2019 09:30

Mathematics, 11.07.2019 09:30

Physics, 11.07.2019 09:30



Mathematics, 11.07.2019 09:30

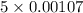 = 0.0053 mg)
= 0.0053 mg) 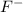 potential will change into -0.2446 V. Hence, potential created by 0.00535 mg
potential will change into -0.2446 V. Hence, potential created by 0.00535 mg 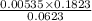 )
)