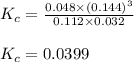Chemistry, 19.03.2020 07:08 xMABRYx1991
Steam reforming of methane ( ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a tank with of methane gas and of water vapor at . He then raises the temperature, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be . Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

You know the right answer?
Steam reforming of methane ( ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrog...
Questions




Mathematics, 20.04.2021 04:10


Mathematics, 20.04.2021 04:10

Chemistry, 20.04.2021 04:10



Mathematics, 20.04.2021 04:10

History, 20.04.2021 04:10

History, 20.04.2021 04:10

Mathematics, 20.04.2021 04:10


History, 20.04.2021 04:10

Mathematics, 20.04.2021 04:10

Mathematics, 20.04.2021 04:10

Biology, 20.04.2021 04:10


Mathematics, 20.04.2021 04:10

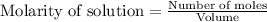
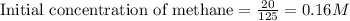
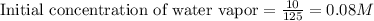
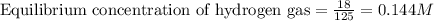
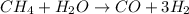
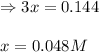
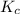 for above equation follows:
for above equation follows:![K_c=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0553/6848/498b4.png)