
Chemistry, 19.03.2020 06:48 JohnStewart714
Compute the equilibrium constant, K, at 25 ∘C for the reaction between Ni2+(aq) and Zn(s), which form Ni(s) and Zn2+(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1
You know the right answer?
Compute the equilibrium constant, K, at 25 ∘C for the reaction between Ni2+(aq) and Zn(s), which for...
Questions


Mathematics, 18.09.2019 16:30


Physics, 18.09.2019 16:30





History, 18.09.2019 16:30


Social Studies, 18.09.2019 16:30



Computers and Technology, 18.09.2019 16:30


Mathematics, 18.09.2019 16:30



Biology, 18.09.2019 16:30

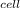 =
= 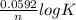
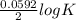
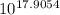 = 2.04×10¹⁷.
= 2.04×10¹⁷. 



