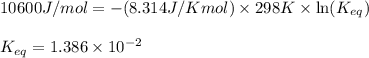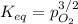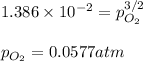Consider the decomposition of a metal oxide to its elements, where M represents a generic metal.
M203(s)---> 2M(s) + 3/2 O2(g) info given for Gf(kJ/mol): M203= -10.60 M(s)=0 O2(g)= 0 1.) what is the standard change in Gibbs energy for rxn as written in forward direction? (kJ/mol)
2.) What is the equilibrium constant (K) of this rxn, as written in forward direction at 298K?
3.) What is the equilibrium pressure of O2(g) over M(s) at 298K? (atm)
For 1.) I got 10.6 kJ/mol and this is correct For 2.) I got K= -4.28 and it marked me wrong. Now I got an answer of .01387. Is this correct???
For 3.) I got PO2= 0.0016 atm and it marked me wrongI don't know how it is wrong.
Could you please check #2 and #3 and tell me what I did wrong and what are the answers thanks.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

You know the right answer?
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal.
Questions















History, 30.07.2019 01:00

Social Studies, 30.07.2019 01:00




Biology, 30.07.2019 01:00


![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f_{(product)}]-\sum [n\times \Delta G^o_f_{(reactant)}]](/tpl/images/0553/6408/f2395.png)
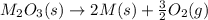
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(M(s))})+(\frac{3}{2}\times \Delta G^o_f_{(O_2(g))})]-[(1\times \Delta G^o_f_{(M_2O_3(s))})]](/tpl/images/0553/6408/d2fa8.png)
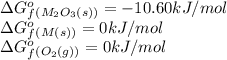
![\Delta G^o_{rxn}=[(2\times (0))+(\frac{3}{2}\times (0))]-[(1\times (-10.60))]\\\\\Delta G^o_{rxn}=10.60kJ/mol](/tpl/images/0553/6408/a75a5.png)
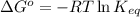
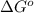 = Standard Gibbs free energy = 10.60 kJ/mol = 10600 J/mol (Conversion factor: 1 kJ = 1000 J )
= Standard Gibbs free energy = 10.60 kJ/mol = 10600 J/mol (Conversion factor: 1 kJ = 1000 J )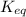 = equilibrium constant = ?
= equilibrium constant = ?