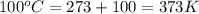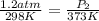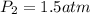Chemistry, 19.03.2020 06:38 ochoanene822
A sample of air occupies 3.8 L when the pressure is 1.2 atm and temperature at 25 Celsius. What pressure( in atm) will the gas have at 100 Celsius when the volum is held constant?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
A sample of air occupies 3.8 L when the pressure is 1.2 atm and temperature at 25 Celsius. What pres...
Questions











Arts, 05.03.2020 05:22









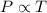
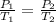
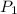 = initial pressure of gas = 1.2 atm
= initial pressure of gas = 1.2 atm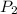 = final pressure of gas = ?
= final pressure of gas = ?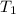 = initial temperature of gas =
= initial temperature of gas = 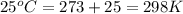
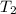 = final temperature of gas =
= final temperature of gas =