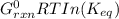Chemistry, 19.03.2020 03:02 nadinealonzo6121
A critical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, ATP, to adenosine diphosphate, ADP, as described by the reaction ATP ( aq ) + H 2 O ( l ) ⟶ ADP ( aq ) + HPO 2 − 4 ( aq ) for which Δ G ∘ rxn = − 30.5 kJ/mol at 37.0 °C and pH 7.0. Calculate the value of Δ G rxn in a biological cell in which [ ATP ] = 5.0 mM, [ ADP ] = 0.20 mM, and [ HPO 2 − 4 ] = 5.0 mM.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
A critical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Mathematics, 10.03.2020 02:59

Mathematics, 10.03.2020 02:59

Computers and Technology, 10.03.2020 02:59

















Mathematics, 10.03.2020 03:00


![K_{eq}= \frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0553/4313/cfc41.png)
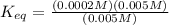
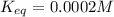
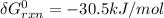 to J/mol; we have:
to J/mol; we have: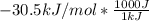
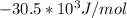
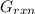 can be calculated as:
can be calculated as: