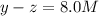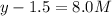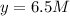Chemistry, 19.03.2020 03:07 DVM117x017
An initial mixture of nitrogen gas and hydrogen gas is reacted in a rigid container at a certain temperature by the reaction
3H2(g) +N2(g) <=> 2NH3(g)
At equilibrium the concentrations are [H2]=5.0 M, [N2]= 8.0M, [NH3]=3.0M
What were the concentrations of nitrogen gas and hydrogen gas that were reacted initially.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2


Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
An initial mixture of nitrogen gas and hydrogen gas is reacted in a rigid container at a certain tem...
Questions


Mathematics, 16.11.2020 20:30


Chemistry, 16.11.2020 20:30

Arts, 16.11.2020 20:30

Business, 16.11.2020 20:30


Spanish, 16.11.2020 20:30


Business, 16.11.2020 20:30

English, 16.11.2020 20:30

Mathematics, 16.11.2020 20:30

Biology, 16.11.2020 20:30

Arts, 16.11.2020 20:30


Biology, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40


Social Studies, 16.11.2020 20:40

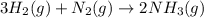
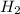 =
= 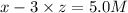
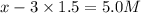
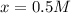
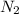 =
=