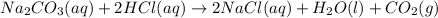A student combined two colorless aqueous solutions. One of the solutions contained Na2CO3 as the solute and the other contained HCl . The chemical reaction that took place is represented by the equation above. What experimental result would be evidence that a chemical reaction took place when the solutions were combined

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 19:00
How can evidence from an experiment be explained in her relationship to the hypothesis? a.as a prediction b.as a question c.as an in inference d.as a conclusion
Answers: 2

Chemistry, 23.06.2019 19:30
⁉️how many kj of energy would be needed to convert 150. g of ammonia to vapor at its boiling point? ⁉️(ammonia’s heat of vaporization is 1.38 kj/g
Answers: 3
You know the right answer?
A student combined two colorless aqueous solutions. One of the solutions contained Na2CO3 as the sol...
Questions

Mathematics, 29.04.2021 04:00

Mathematics, 29.04.2021 04:00


Mathematics, 29.04.2021 04:00


Computers and Technology, 29.04.2021 04:00


Mathematics, 29.04.2021 04:00


Mathematics, 29.04.2021 04:00

English, 29.04.2021 04:00




Mathematics, 29.04.2021 04:00

Mathematics, 29.04.2021 04:00



Mathematics, 29.04.2021 04:00

Mathematics, 29.04.2021 04:00

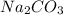 and
and 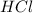 as a solute will be combined, the reaction will be as follows,
as a solute will be combined, the reaction will be as follows,