
Consider a saturated aqueous solution of Ag2S that contains some excess solid Ag2S at the bottom. Which of the following statements is/are true? Adding AgNO3 to the solution will cause more Ag2S to dissolve. Adding some more Ag2S to the solution will cause more Ag2S to dissolve. Adding some water to the solution will cause more Ag2S to dissolve.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
Consider a saturated aqueous solution of Ag2S that contains some excess solid Ag2S at the bottom. Wh...
Questions





Mathematics, 08.03.2021 19:40






Computers and Technology, 08.03.2021 19:40

Mathematics, 08.03.2021 19:40






History, 08.03.2021 19:40


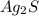 to dissolve is a true statement.
to dissolve is a true statement.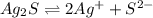
![K_{sp}=[Ag^{+}]^{2}[S^{2-}]](/tpl/images/0553/3669/0192a.png)
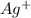 increases when
increases when 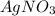 is added into solution. But value of
is added into solution. But value of 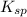 is constant at a certain temperature. Hence to keep
is constant at a certain temperature. Hence to keep 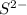 to produce more
to produce more 

