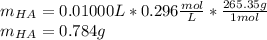Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Thiamine hydrochloride (C12H18ON4SCl2) is a water-soluble form of thiamine (vitamin B1; Ka = 3.37×10...
Questions

Mathematics, 24.05.2020 10:57

Mathematics, 24.05.2020 10:57


Mathematics, 24.05.2020 10:57




History, 24.05.2020 10:57

Mathematics, 24.05.2020 10:57


Mathematics, 24.05.2020 10:57

Biology, 24.05.2020 10:57


Mathematics, 24.05.2020 10:57


Chemistry, 24.05.2020 10:57

Physics, 24.05.2020 10:57


Biology, 24.05.2020 10:57

English, 24.05.2020 10:57

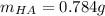
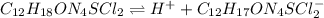
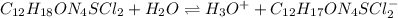
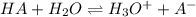
![pH=pKa+log(\frac{[A^-]}{[HA]} )](/tpl/images/0553/3571/4a01a.png)
![log(\frac{[A^-]}{[HA]} )=3.50-[-log(3.37x 10^{-7})]=3.50-6.47=-2.97}\\\\\frac{[A^-]}{[HA]} =10^{-2.97}=1.07x10^{-3}](/tpl/images/0553/3571/b4cdb.png)
![[A^-]=1.07x10^{-3}}[HA]](/tpl/images/0553/3571/1acb8.png)
![[H]^+=[A^-]=10^{-pH}=10^{-3.50}=3.16x10^{-4}M](/tpl/images/0553/3571/d3a0e.png)
![[HA]=\frac{3.16x10^{-4}M}{1.07x10^{-3}} =0.296M](/tpl/images/0553/3571/7981c.png)