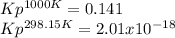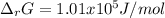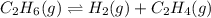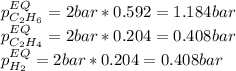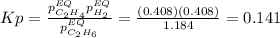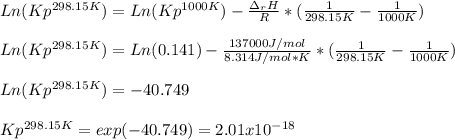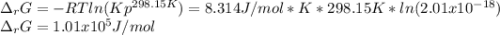Consider the equilibrium
At 1000 K and a const. total pressure of 2 bar, C2H6 is introd...

Consider the equilibrium
At 1000 K and a const. total pressure of 2 bar, C2H6 is introduced into a reaction vessel. the total pressure is held const. at 2 bar and at equilibrium the composition of the mixture in mole percent is H2(g): 20.4%, C2H4 (g): 20.4%, and C2H6 (g): 59.2%.
Calculate Kp at 1000 K
if ΔH of reaction = 137 kJ/mol, calculate the value of Kp at 298.15K
Calculate ΔG of reaction for this reaction at 298.15 K

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
Questions





Mathematics, 19.01.2021 18:50






Business, 19.01.2021 18:50

Biology, 19.01.2021 18:50

Chemistry, 19.01.2021 18:50

Biology, 19.01.2021 18:50


Mathematics, 19.01.2021 18:50


Computers and Technology, 19.01.2021 18:50