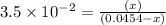Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10−2 If you start with 25.0 mLmL of a 0.909 MM solution of NaIO4NaIO4, and then dilute it with water to 500.0 mLmL, what is the concentration of H4IO−6H4IO6− at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1


Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10...
Questions


History, 20.10.2020 23:01

English, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01





Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01



Mathematics, 20.10.2020 23:01

English, 20.10.2020 23:01


Arts, 20.10.2020 23:01

English, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

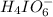 at equilibrium is, 0.00154 M
at equilibrium is, 0.00154 M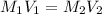
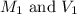 are the initial molarity and volume of
are the initial molarity and volume of 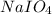 .
.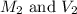 are the final molarity and volume of diluted
are the final molarity and volume of diluted 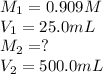
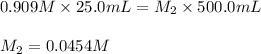
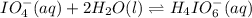
![K_c=\frac{[H_4IO_6^-]}{[IO_4^-]}](/tpl/images/0553/1589/ffca5.png)