Chemistry, 19.03.2020 00:53 zoeyandblaze
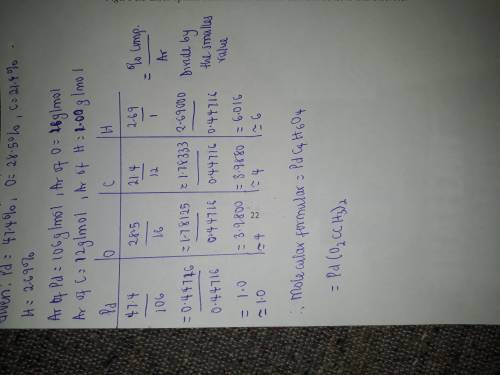
A compound has the percent composition 47.40% Pd, 28.50% O, 21.40% C, and 2.69% H. Based on this information, which molecular formulas could represent the compound? (Choose more than one answer)
PdO2C2H3
Pd(O2CCH3)2
Pd(O2C2H3)3
PdO4C2H9
Pd2C8H12O8

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
A compound has the percent composition 47.40% Pd, 28.50% O, 21.40% C, and 2.69% H. Based on this inf...
Questions


History, 19.10.2020 03:01


Physics, 19.10.2020 03:01




Geography, 19.10.2020 03:01

Mathematics, 19.10.2020 03:01


English, 19.10.2020 03:01




Advanced Placement (AP), 19.10.2020 03:01

Mathematics, 19.10.2020 03:01

History, 19.10.2020 03:01


Biology, 19.10.2020 03:01