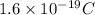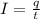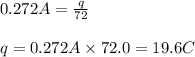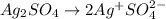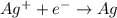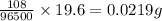Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. In essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. Suppose a current of is passed through an electroplating cell with an aqueous solution of in the cathode compartment for seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) laye...
Questions

Biology, 17.07.2020 06:01

English, 17.07.2020 06:01

Mathematics, 17.07.2020 06:01

Mathematics, 17.07.2020 06:01

Mathematics, 17.07.2020 06:01




English, 17.07.2020 06:01

English, 17.07.2020 06:01


Arts, 17.07.2020 06:01

Biology, 17.07.2020 06:01


Biology, 17.07.2020 06:01

Mathematics, 17.07.2020 06:01

Mathematics, 17.07.2020 06:01

History, 17.07.2020 06:01

Mathematics, 17.07.2020 06:01

History, 17.07.2020 06:01

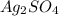 in the cathode compartment for 72 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Be sure your answer has a unit symbol and the correct number of significant digits.
in the cathode compartment for 72 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Be sure your answer has a unit symbol and the correct number of significant digits.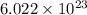 number of particles.
number of particles.