
Chemistry, 18.03.2020 18:32 laceysmith2i023
Al(OH)3 + OH- → Al(OH)4- Identify whether this reaction can be classified as a Bronsted-Lowry and/or Lewis acid/base reaction. Group of answer choices Only Bronsted-Lowry Only Lewis Both Bronsted-Lowry and Lewis Neither

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Al(OH)3 + OH- → Al(OH)4- Identify whether this reaction can be classified as a Bronsted-Lowry and/or...
Questions


Mathematics, 18.03.2021 03:00

History, 18.03.2021 03:00



Chemistry, 18.03.2021 03:00

Physics, 18.03.2021 03:00



Business, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00



Mathematics, 18.03.2021 03:00

Geography, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

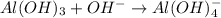
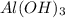 is a Lewis acid because it is an electron deficient species that means, it accepts electron pairs.
is a Lewis acid because it is an electron deficient species that means, it accepts electron pairs.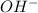 is a Lewis base because it is an electron rich species that means, it donates electron pairs.
is a Lewis base because it is an electron rich species that means, it donates electron pairs.

