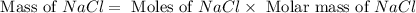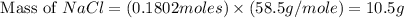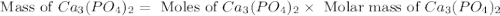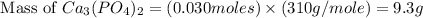Chemistry, 18.03.2020 18:46 yuvraj2298
Calculate the mass of calcium phosphate and the mass of sodium chloride that could be formed when a solution containing 12.00g of sodium phosphate is added to a solution containing 10.0g of calcium chloride

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Calculate the mass of calcium phosphate and the mass of sodium chloride that could be formed when a...
Questions

Mathematics, 12.08.2020 04:01






English, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01






Spanish, 12.08.2020 04:01




Computers and Technology, 12.08.2020 04:01


History, 12.08.2020 04:01

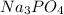 = 12.00 g
= 12.00 g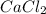 = 10.0 g
= 10.0 g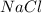 = 58.5 g/mol
= 58.5 g/mol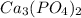 = 310 g/mol
= 310 g/mol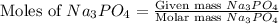
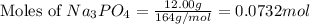

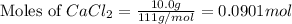
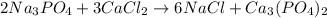
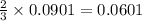 moles of
moles of 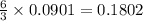 mole of
mole of 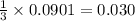 mole of
mole of