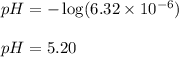Your job is to determine the concentration of ammonia in a commercial window cleaner. In the titration of a 25.0 mL sample of the cleaner, the equivalence point is reached after 23.8 mL of 0.164 M HCl has been added. What is the initial concentration of ammonia in the solution? What is the pH of the solution at the equivalence point?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
Your job is to determine the concentration of ammonia in a commercial window cleaner. In the titrati...
Questions

History, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01




English, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01



History, 12.08.2020 08:01


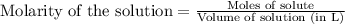 .....(1)
.....(1)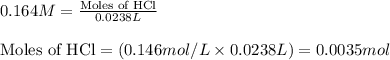
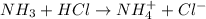
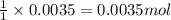 of ammonia
of ammonia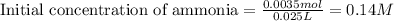
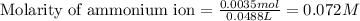
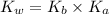
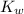 = Ionic product of water =
= Ionic product of water = 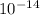
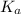 = Acid dissociation constant
= Acid dissociation constant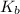 = Base dissociation constant =
= Base dissociation constant = 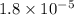
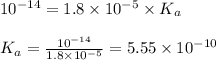
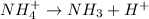
![K_a=\frac{[NH_3][H^+]}{[NH_4^+]}](/tpl/images/0551/3928/47aaa.png)
![[NH_3]=[H^+]=x](/tpl/images/0551/3928/ae0a0.png)
![[NH_4^+]=0.072M](/tpl/images/0551/3928/583ca.png)
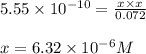
![pH=-\log[H^+]](/tpl/images/0551/3928/cf945.png)
![[H^+]=6.32\times 10^{--6}M](/tpl/images/0551/3928/81536.png)