Chemistry, 17.03.2020 22:05 ellareynolds2337
How many liters of hydrogen gas will be produced at 280.0 K and 96.0 kPa if 40.0 grams of sodium react with excess hydrochloric acid? Write the balanced equation first.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
How many liters of hydrogen gas will be produced at 280.0 K and 96.0 kPa if 40.0 grams of sodium rea...
Questions

Mathematics, 06.03.2021 05:40


Arts, 06.03.2021 05:40

Spanish, 06.03.2021 05:40


Mathematics, 06.03.2021 05:40




History, 06.03.2021 05:40




Chemistry, 06.03.2021 05:40



Mathematics, 06.03.2021 05:40


Physics, 06.03.2021 05:40

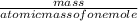
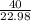
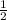 =
=