
Chemistry, 17.03.2020 21:23 kaiyah2021
Space vehicles use solid lithium hydroxide to remove exhaled carbon dioxide according to the equation: 2LiOH + CO 2 Li 2 CO 3 + H 2 O. Determine the mass of carbon dioxide removed if the space vehicle carries 42.0 mol of LiOH.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Space vehicles use solid lithium hydroxide to remove exhaled carbon dioxide according to the equatio...
Questions




History, 04.02.2022 14:00

History, 04.02.2022 14:00

English, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00


French, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00



Social Studies, 04.02.2022 14:00

Spanish, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00




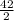 = 21moles of CO₂
= 21moles of CO₂

