
Chemistry, 17.03.2020 20:48 sgslayerkingminecraf
A mixture of CO2 and Kr weighs 31.7 g and exerts a pressure of 0.665 atm in its container. Since Kr is expensive, you wish to recover it from the mixture. After the CO2 is completely removed by absorption with NaOH(s), the pressure in the container is 0.309 atm.
(a) How many grams of CO2 were originally present?
(b) How many grams of Kr can you recover?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
A mixture of CO2 and Kr weighs 31.7 g and exerts a pressure of 0.665 atm in its container. Since Kr...
Questions





Social Studies, 24.10.2019 16:43




Computers and Technology, 24.10.2019 16:43











Mathematics, 24.10.2019 16:43

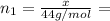
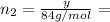
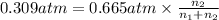
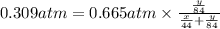
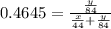 ..[2]
..[2]

