
Chemistry, 17.03.2020 20:00 davfar334p47luq
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially by acidifying the solution. Write all the relevant equilibrium equations and explain why adding acid will increase the solubility of calcium carbonate.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 10:10
Which orbitals form a pi bond? a. the s orbital and three p orbitals b. the s orbital and two p orbitals c. overlapping p orbitals d. overlapping hybrid orbitals
Answers: 2

Chemistry, 23.06.2019 14:30
When does phenolphthalein turn pink? in the presence of a base in the presence of an acid when it is in a neutral solution when it is reacting with a metal
Answers: 1
You know the right answer?
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially...
Questions

Mathematics, 26.10.2020 03:30



Chemistry, 26.10.2020 03:30

Chemistry, 26.10.2020 03:30


Physics, 26.10.2020 03:30


Physics, 26.10.2020 03:30

Mathematics, 26.10.2020 03:30

History, 26.10.2020 03:30


Chemistry, 26.10.2020 03:30


English, 26.10.2020 03:30





History, 26.10.2020 03:30

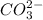 is converted to
is converted to 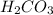 in acidic solution.
in acidic solution.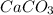 dissociates in solution to produce
dissociates in solution to produce 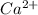 and
and 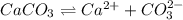
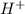 and gets converted to
and gets converted to 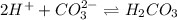 So,
So, 

