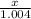Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
Calculate the mass of hydrogen formed when 27 g of aluminum reacts with excess hydrochloric acid acc...
Questions


Biology, 31.03.2020 18:27

Mathematics, 31.03.2020 18:27


Social Studies, 31.03.2020 18:27


Mathematics, 31.03.2020 18:27

Biology, 31.03.2020 18:27



History, 31.03.2020 18:27

Mathematics, 31.03.2020 18:27

Mathematics, 31.03.2020 18:27


Mathematics, 31.03.2020 18:27

History, 31.03.2020 18:27


Mathematics, 31.03.2020 18:27


Business, 31.03.2020 18:27

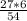 = 3 g of H₂.
= 3 g of H₂.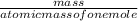
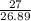
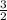 =
=