
Chemistry, 17.03.2020 19:24 bobbyhill24
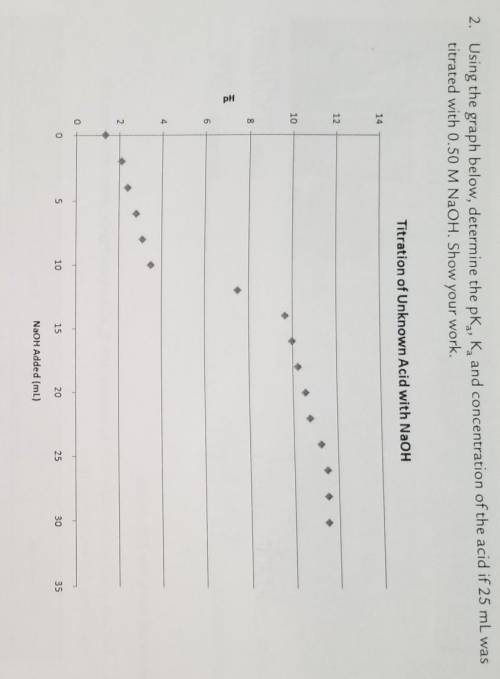
Using the graph below, determine the pKa, Ka, and concentration of the acid if 25 mL was titrated with 0.50 M NaOH. Show your work.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
Using the graph below, determine the pKa, Ka, and concentration of the acid if 25 mL was titrated wi...
Questions

Geography, 05.05.2020 00:07



English, 05.05.2020 00:07

History, 05.05.2020 00:07

Mathematics, 05.05.2020 00:07


Mathematics, 05.05.2020 00:07



Mathematics, 05.05.2020 00:07


Mathematics, 05.05.2020 00:07



Mathematics, 05.05.2020 00:07

History, 05.05.2020 00:07






