
Chemistry, 17.03.2020 17:40 tinktkinkdavis7340
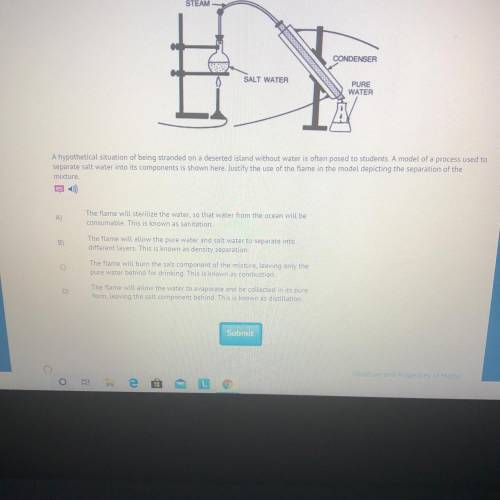
A hypothetical situation of being stranded on a deserted island without water is often posed to students. A model of a process used to
separate salt water into its components is shown here. Justify the use of the flame in the model depicting the separation of the
mixture.
The flame will sterilize the water, so that water from the ocean will be
consumable. This is known as sanitation.
The flame will allow the pure water and salt water to separate into
different layers. This is known as density separation.
The flame will burn the salt component of the mixture, leaving only the
pure water behind for drinking. This is known as combustion.
The flame will allow the water to evaporate and be collected in its pure
form, leaving the salt component behind. This is known as distillation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
A hypothetical situation of being stranded on a deserted island without water is often posed to stud...
Questions

English, 09.01.2020 04:31

History, 09.01.2020 04:31

Physics, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31



Biology, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31

Computers and Technology, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31


English, 09.01.2020 04:31


Mathematics, 09.01.2020 04:31

Biology, 09.01.2020 04:31

Social Studies, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31



