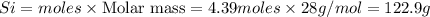The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
...

The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
2Cr2O3(s)+3Si(s) > 4Cr(l)+3SiO2(s)
The reaction is begun with 161.00 g of Si and 139.00 g of Cr2O3.
How many grams of the excess reactant is left after the reaction is complete?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1
You know the right answer?
Questions

Mathematics, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30


History, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

Biology, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30



Biology, 17.12.2020 07:30


Health, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30


Mathematics, 17.12.2020 07:30

Biology, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

Health, 17.12.2020 07:30

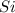 will be left from the given masses of both reactants.
will be left from the given masses of both reactants.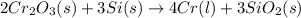
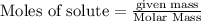
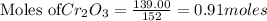
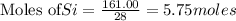
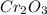 require 3 moles of
require 3 moles of 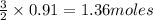 of
of