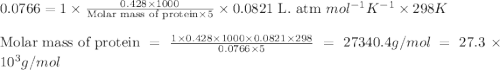Chemistry, 17.03.2020 05:41 meramera50
428. mg of an unknown protein are dissolved in enough solvent to make of solution. The osmotic pressure of this solution is measured to be at . Calculate the molar mass of the protein. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
428. mg of an unknown protein are dissolved in enough solvent to make of solution. The osmotic press...
Questions

Physics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00







English, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Biology, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

English, 11.02.2021 14:00

History, 11.02.2021 14:00

Chemistry, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00

Social Studies, 11.02.2021 14:00

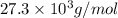
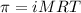
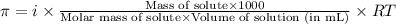
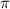 = osmotic pressure of the solution = 0.0766 atm
= osmotic pressure of the solution = 0.0766 atm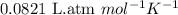
![25^oC=[273+25]=298K](/tpl/images/0550/3624/6a9f9.png)