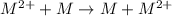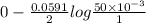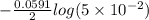Chemistry, 17.03.2020 05:25 asaadasaadasaad6524
A certain metal M forms a soluble sulfate salt MSO, Suppose the left half cell of a galvanic cell apparatus is filled with a 3.00 M solution of MSO, and the ight half cell with a 30.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C lf right 410 Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
A certain metal M forms a soluble sulfate salt MSO, Suppose the left half cell of a galvanic cell ap...
Questions

Chemistry, 06.06.2021 16:40


History, 06.06.2021 16:50

Mathematics, 06.06.2021 16:50


Biology, 06.06.2021 16:50

Chemistry, 06.06.2021 16:50


Arts, 06.06.2021 16:50


English, 06.06.2021 16:50


Social Studies, 06.06.2021 16:50

Computers and Technology, 06.06.2021 16:50

English, 06.06.2021 16:50


Mathematics, 06.06.2021 16:50

Mathematics, 06.06.2021 16:50

Social Studies, 06.06.2021 16:50

Social Studies, 06.06.2021 16:50

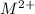 , reduction will take place. As, cathode has a positive charge and it will be placed on left hand side.
, reduction will take place. As, cathode has a positive charge and it will be placed on left hand side.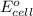 = 0 and the general reaction equation is as follows.
= 0 and the general reaction equation is as follows.