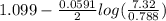A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: Cu2 (aq)+Zn () Cu (s)+Zn2(aq) Suppose the cell is prepared with 0.788 MCu2 in one half-cell and 7.32 M Zn2 in the other Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: Cu2 (aq)+Zn (...
Questions


Computers and Technology, 21.01.2021 18:30

Social Studies, 21.01.2021 18:30




English, 21.01.2021 18:30

Geography, 21.01.2021 18:30

History, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30


Biology, 21.01.2021 18:30



Mathematics, 21.01.2021 18:30

Social Studies, 21.01.2021 18:30

English, 21.01.2021 18:30

History, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30

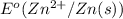 = -0.7618 V
= -0.7618 V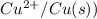 and anode is ().
and anode is ().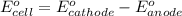
![E^{o} - (\frac{2.303 \times RT}{nF}) log {\frac{[Zn^{2+}]}^{1}{[Cu^{2+}]^{1}}](/tpl/images/0550/3152/96c78.png)
![E^{o} - (\frac{2.303 \times RT}{nF}) log {\frac{[Zn^{2+}]}^{1}{[Cu^{2+}]}^{1}](/tpl/images/0550/3152/d6ff2.png)
![E^{o} - (\frac{0.0591}{n}) log \frac{[Zn^{2+}]}^{1}{[Cu^{2+}]}^{1}](/tpl/images/0550/3152/6368f.png)