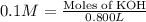Chemistry, 17.03.2020 04:19 theodisb8440
A chemist must prepare 800ml of potassium hydroxide solution with a pH 13 of at 25. He will do this in three steps:
Fill a volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1


Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
A chemist must prepare 800ml of potassium hydroxide solution with a pH 13 of at 25. He will do this...
Questions

Biology, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

English, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Spanish, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01


Mathematics, 09.09.2020 07:01

Physics, 09.09.2020 07:01

English, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Mathematics, 09.09.2020 07:01

Spanish, 09.09.2020 07:01

![pOH=-\log[OH^-]](/tpl/images/0550/1622/fe336.png)
![1=-\log[OH^-]](/tpl/images/0550/1622/3f830.png)
![[OH^-]=0.1 M](/tpl/images/0550/1622/e907d.png)
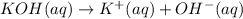
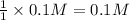 of KOH
of KOH![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0550/1622/0dac6.png)