
Chemistry, 17.03.2020 03:18 violetagamez2
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0300 M Ag + ( aq ) . What will be the concentration of Ca 2 + ( aq ) when Ag 2 SO 4 ( s ) begins to precipitate? Solubility-product constants, K sp , can be found in the chempendix.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0300 M Ag + ( a...
Questions

Biology, 20.05.2021 02:30


English, 20.05.2021 02:30

Mathematics, 20.05.2021 02:30

Health, 20.05.2021 02:30

World Languages, 20.05.2021 02:30



Mathematics, 20.05.2021 02:30




Mathematics, 20.05.2021 02:30

Mathematics, 20.05.2021 02:30

History, 20.05.2021 02:30

Mathematics, 20.05.2021 02:30



Mathematics, 20.05.2021 02:30

Mathematics, 20.05.2021 02:40

![[Ca^{2+}]](/tpl/images/0550/0535/17576.png) ion is, 0.00371 M
ion is, 0.00371 M![[SO_4^{2-}]](/tpl/images/0550/0535/43b69.png) ion.
ion.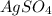 will be:
will be: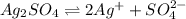
![K_{sp}=[Ag^{+}]^2[SO_4^{2-}]](/tpl/images/0550/0535/80963.png)
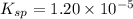
![1.20\times 10^{-5}=(0.0300)^2\times [SO_4^{2-}]](/tpl/images/0550/0535/d8931.png)
![[SO_4^{2-}]=0.0133M](/tpl/images/0550/0535/cd404.png)
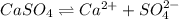
![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0550/0535/958f2.png)
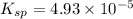
![4.93\times 10^{-5}=[Ca^{2+}]\times (0.0133)](/tpl/images/0550/0535/7fa1a.png)
![[Ca^{2+}]=0.00371M](/tpl/images/0550/0535/7d329.png)


