
Chemistry, 17.03.2020 01:09 haileeattaway
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO2 + NO2 → NO + NO3 step 2: NO3 + CO → NO2 + CO2 The experimental rate law is rate = k[NO2]2 (a) Write the equation for the overall reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO...
Questions

Mathematics, 13.04.2021 22:50



Mathematics, 13.04.2021 22:50

Chemistry, 13.04.2021 22:50

Mathematics, 13.04.2021 22:50






Mathematics, 13.04.2021 22:50



English, 13.04.2021 22:50




History, 13.04.2021 22:50

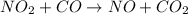
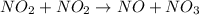
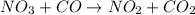
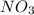 is produced in the first step and subsequently consumed in second step. Hence,
is produced in the first step and subsequently consumed in second step. Hence, 


