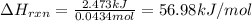In a constant‑pressure calorimeter, 70.0 mL of 0.310 M Ba ( OH ) 2 was added to 70.0 mL of 0.620 M HCl . The reaction caused the temperature of the solution to rise from 21.12 ∘ C to 25.34 ∘ C. If the solution has the same density and specific heat as water, what is heat absorbed by the solution? Assume that the total volume is the sum of the individual volumes. (And notice that the answer is in kJ).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
In a constant‑pressure calorimeter, 70.0 mL of 0.310 M Ba ( OH ) 2 was added to 70.0 mL of 0.620 M H...
Questions

Biology, 14.11.2019 00:31




Mathematics, 14.11.2019 00:31

English, 14.11.2019 00:31


Mathematics, 14.11.2019 00:31



Computers and Technology, 14.11.2019 00:31


Mathematics, 14.11.2019 00:31

English, 14.11.2019 00:31

Chemistry, 14.11.2019 00:31

Chemistry, 14.11.2019 00:31


World Languages, 14.11.2019 00:31


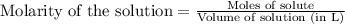 .....(1)
.....(1)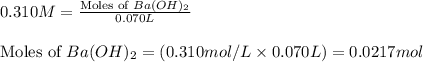
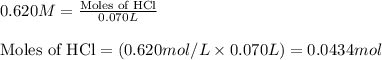
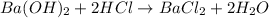
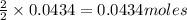 of water
of water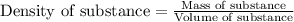
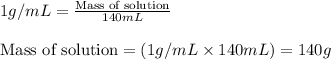
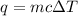
 = change in temperature =
= change in temperature = 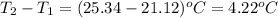
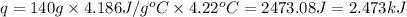
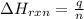
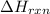 = enthalpy change of the reaction
= enthalpy change of the reaction