Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

You know the right answer?
A solution of phosphoric acid was made by dissolving 10.0 g of H3PO4 in 100.0 mL of water. The resul...
Questions


Mathematics, 20.01.2021 18:20







English, 20.01.2021 18:20

Business, 20.01.2021 18:20

Mathematics, 20.01.2021 18:20

Social Studies, 20.01.2021 18:20



Mathematics, 20.01.2021 18:20


Mathematics, 20.01.2021 18:20

Mathematics, 20.01.2021 18:20


Mathematics, 20.01.2021 18:20

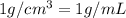
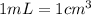
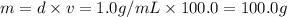
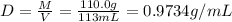
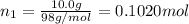
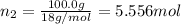
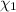
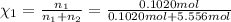
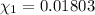
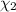
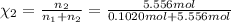
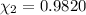
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0549/6266/0dac6.png)
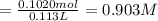
![[Molality]=\frac{\text{Moles of solute}}{\text{Mass of solvent(kg)}}](/tpl/images/0549/6266/71fd2.png)